-40%
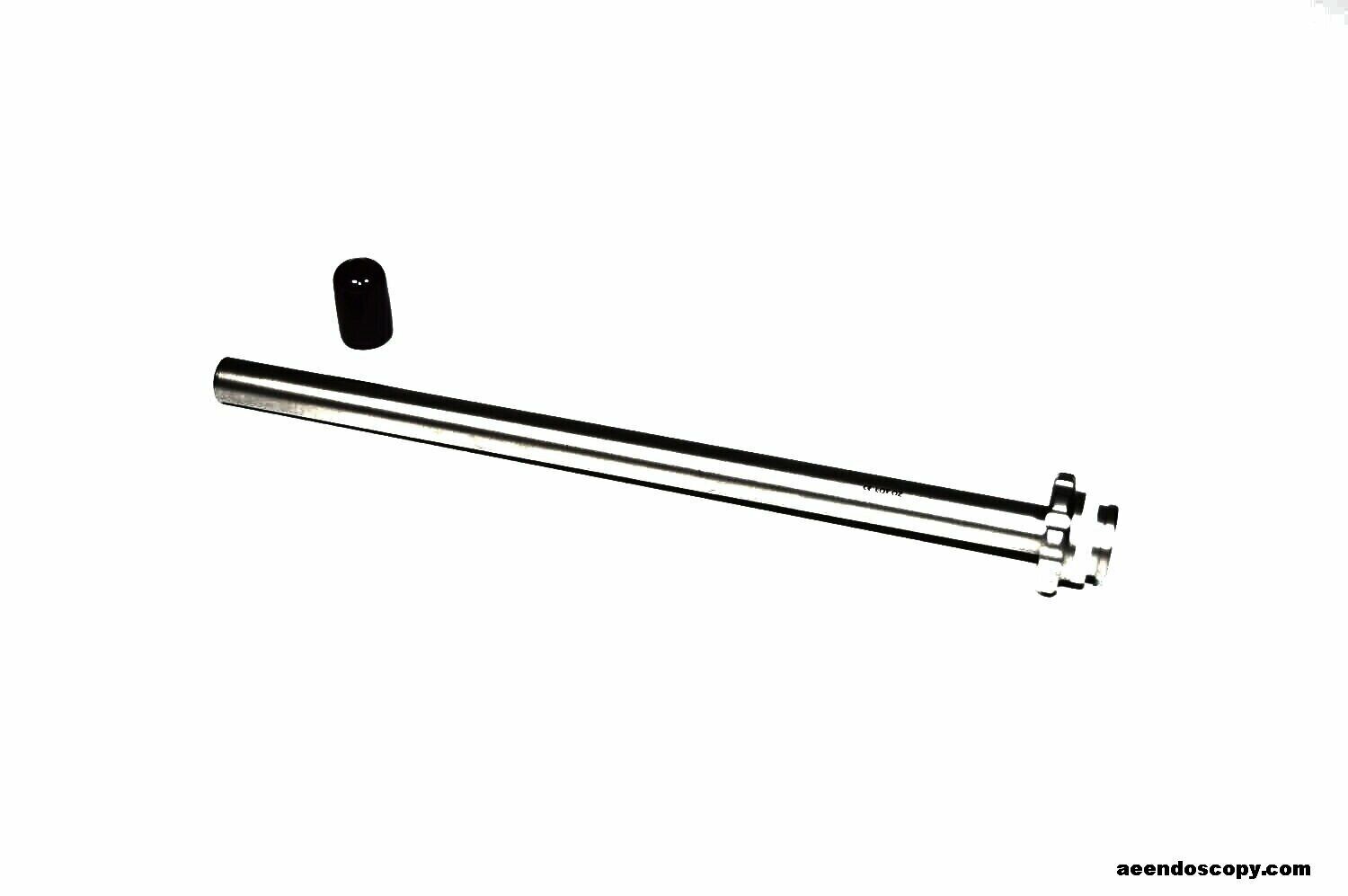
STRYKER Iconix Drill Guide Set 3910-500-563
$ 1894.99
- Description
- Size Guide
Description
STRYKER Iconix Drill Guide Set 3910-500-563This
STRYKER
Iconix Drill Guide Set 3910-500-563 is in great cosmetic and working condition.
What's Included
Stryker
Champion Slider Handle
;
(REF
3910-500-750
);Qty:1
Stryker
ICONIX
;(REF
3910-500-563
);Qty:1
Stryker Iconix
;(REF
3910-500-565
);Qty:1
Stryker Iconix
;(REF
3910-500-562
);Qty:1
Styker Iconix
;(REF
3910-500-564
);Qty:1
Stryker Iconix
2.3MM GUIDE ;(REF
3910-500-557
);Qty:1
Stryker Iconix
1.4 MM GUIDE ;(REF
3910-500-554
);Qty:1
Stryker Iconix
2.3mm GUIDE ;(REF
3910-500-556
);Qty:1
Stryker Iconix
1.4mm GUIDE ;(REF
3910-500-553
);Qty:1
Stryker Iconix
2.3mm Straight Guide ;(REF
3910-500-555
);Qty:1
Stryker Iconix
1.4MM Straight Guide Short ;(REF
3910-500-550
);Qty:1
Stryker Iconix
Straight Guide Short For 2.3mm Anchor
;(REF
3910-500-551
);Qty:1
Stryker Iconix
1.4MM Straight Guide;(REF
3910-500-552
) ;Qty:1
__________________________________________
This device has been carefully reviewed and conforms with eBay’s
Prescription devices policy
.
To the best of our knowledge and abilities, this device has been cleaned and handled in accordance with manufacturer’s instructions.
"The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item."
---
Also per eBay’s Prescription Devices Policy, we must provide the following information about our company for listings of devices that may fall under this FDA guideline:
KenMed Supply, LLC, d.b.a, KenMed Surgical
Casselberry, Florida
[phone removed by eBay] – Office Number