-40%
Storz Sawalhe Morcellator items 20711030, 26711032, etc - Used - UNTESTED
$ 630.95
- Description
- Size Guide
Description
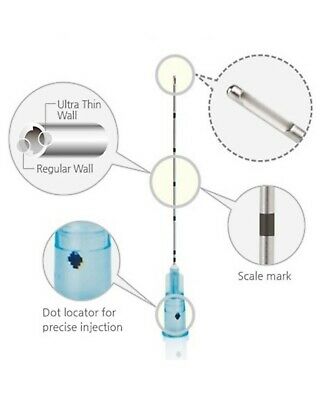
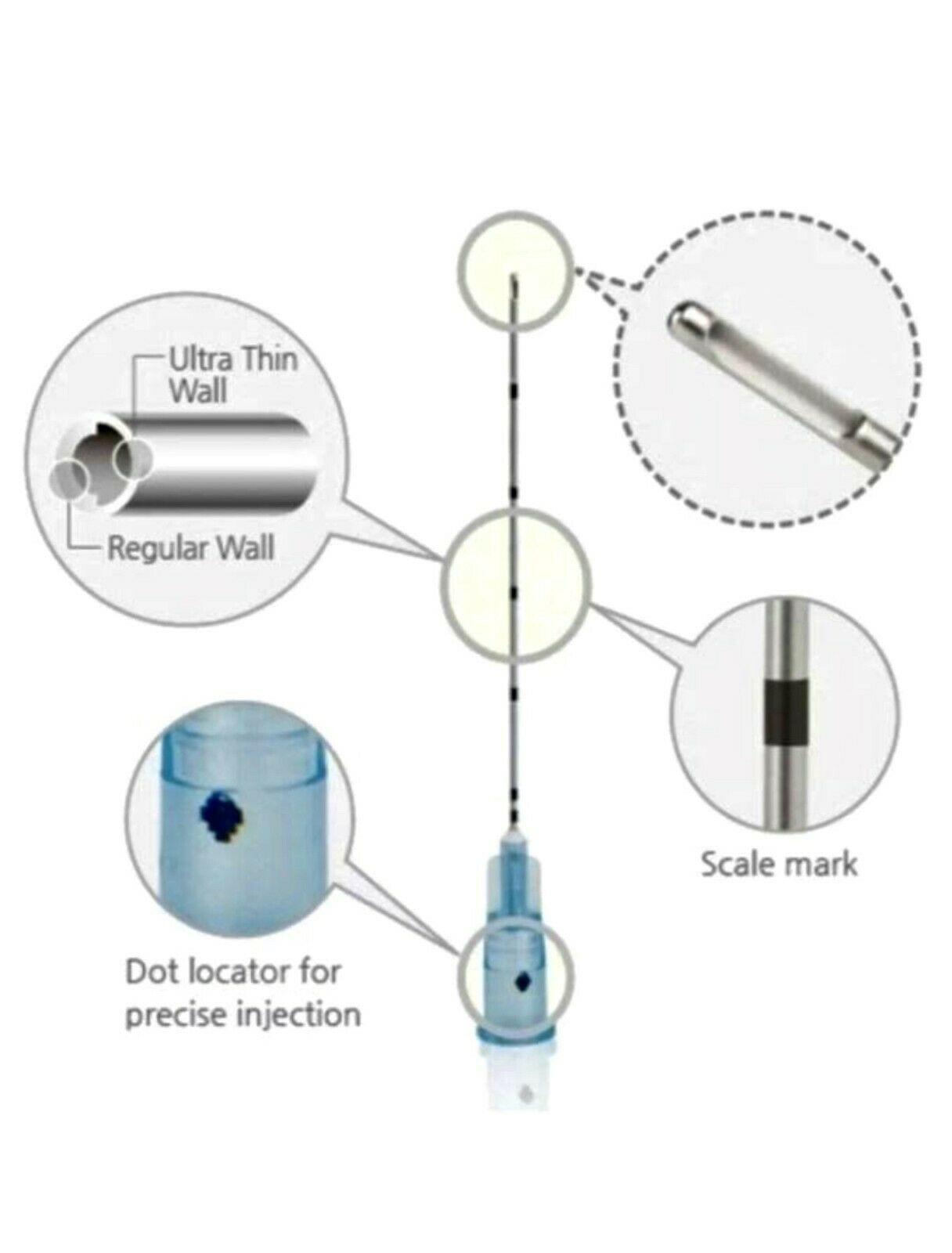
1 - Storz Morcellator pieces including all items seen in photos …Some details on items that had identifying marks are:
1 - 20711030 …
1 - 26711032 …
1 - 26711252 …
1 - P3 …
1 - 26711253 …
This equipment is completely untested and therefore we cannot verify that it works in any shape, way or form.
These pieces are in an original storage case BUT The lid does not appear to be the correct lid in terms of its labeling. The lid to this tray uses the word ‘Resectoscope’, etc.
We are NOT Biomedical Engineers and we have not repaired, tested, refurbished, or rebuilt this equipment. We are not guaranteeing it for any specific purpose implied or direct. No Returns. It is up to the Buyer to immediately disinfect the equipment and have a qualified person test it for safety for all people that could be involved in its use and to make sure that it is meeting manufacturer’s original specifications.
Disclaimer
: This item is sold as is, no warranties implied. The item described and/or pictured offered here is in no way certified for, recommended for, or offered for any specific use. The purchaser agrees that the seller shall not be held responsible or liable for any injuries or damages, whether incidental or coincidental, associated in any way with this equipment. It is the sole responsibility of the purchaser to obtain qualified help to ensure the proper functioning, calibration, and safety (mechanical, electrical, etc.) of this equipment. The purchaser by biding on this item, indicates their knowledge of an agreement to the terms of this disclaimer. This item is sold completely as is/ as shown with no warranty either expressed or implied. The sale of this item maybe subject to regulations by the U.S. Food and Drug Administration, state and local regulatory agencies. If you should have questions about legal obligations, you should consult with the U.S. Food and Drug Administration.