-40%
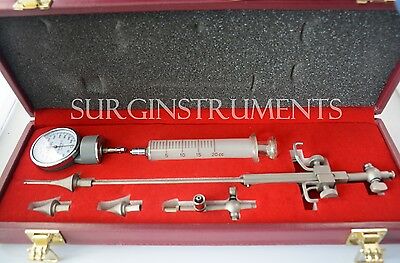
Gyrus ACMI Reusable Universal Stopcock Adapter Luer Lock - 75U Urology OBGYN
$ 23.76
- Description
- Size Guide
Description
We offerFast Shipping
on all orders!
Same Day shipping for completed orders received by 12 pm Eastern Standard Time or next business day of receiving payment (Monday-Friday)
International customers are responsible for any and all customs, import duties, brokerage fees, and taxes. Original shipping charges are non-refundable.
Returns
If you are not 100% satisfied with your purchase, you can return the product and get a full refund or exchange the product within 30 days for some items provided items are not damaged or tampered with.
Customer is responsible for all shipping fees, and initial shipping charges are non-refundable.
DISCLAIMER
Regardless of the origin of the equipment, documentation provided or identification appearing upon the equipment, the equipment described and offered here is in no way certified for, recommended for, or offered for any specific use. The purchaser agrees that the seller shall not be held responsible or liable for any injuries or damages, whether incidental or consequential, associated in any way with the equipment. The purchaser, by bidding on this equipment, indicates their acknowledgment of, and agreement to the terms of this disclaimer.
'"The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item."
If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health. Copy and Paste any of the two links below in the address bar of your web browser.
http://www.fda.gov/cdrh/devadvice
http://www.fda.gov/cdrh/industry/support/index.html
EndocorpUSA
21133 Bridge St
Southfield MI
48033
(248)223-9900